By Mia Rabson in Ottawa
The first COVID-19 vaccine for infants and very young children is now under review by Health Canada.
Moderna Canada President Patricia Gauthier said Friday the company sent an application to the Canadian vaccine regulator late Thursday for a vaccine to protect children between six months and five years old.
"It's now in the hands of Health Canada," she said at an event in Montreal where the company announced plans to build a vaccine production plant.
Health Canada confirmed it began considering the application Friday.
The company said a trial of 6,700 children determined the vaccine was safe and produced a similar antibody response to the one that is seen in adults.
A single dose for children under six is 25 micrograms — one-quarter of the size given to adults and teenagers and half the size used for children ages six to 11.
The application is for the vaccine to be given in two doses, four weeks apart.
The trial took place mainly during the wave of the Omicron variant and the vaccine was less effective at preventing infection in kids than previous trials in adults.
The effectiveness of the vaccine against infection in adults also dropped during the Omicron wave, though the shot maintained excellent protection against serious illness, hospitalization and death.
The company said the vaccine was 51 per cent effective at preventing symptoms in children six months to two years old and 37 per cent effective against symptoms in children two to five years old. It said those are similar results to what was seen in effectiveness against Omicron for adults.
Because children have very low rates of serious illness due to COVID-19, the trial could not determine the impact on preventing serious illness.
Moderna's COVID-19 vaccine was first approved for use on adults in December 2020, for those 12 to 17 in August 2021 and for children aged six to 11 in March.
No vaccine has been authorized in Canada for children younger than five. The Pfizer-BioNTech pediatric vaccine is authorized for children five to 11 in a dose one-third the size given to teenagers and adults.
Its trial for children under five found two doses that are one-tenth the size of the adult dose weren't enough to generate a good enough antibody response. The company expanded the trial to include a third dose.
Results from that trial are expected in May.
Nationally, 40 per cent of children between five and 11 are fully vaccinated, as are 84 per cent of kids and teens between 12 and 17 and 88 per cent of everyone over the age of 18.
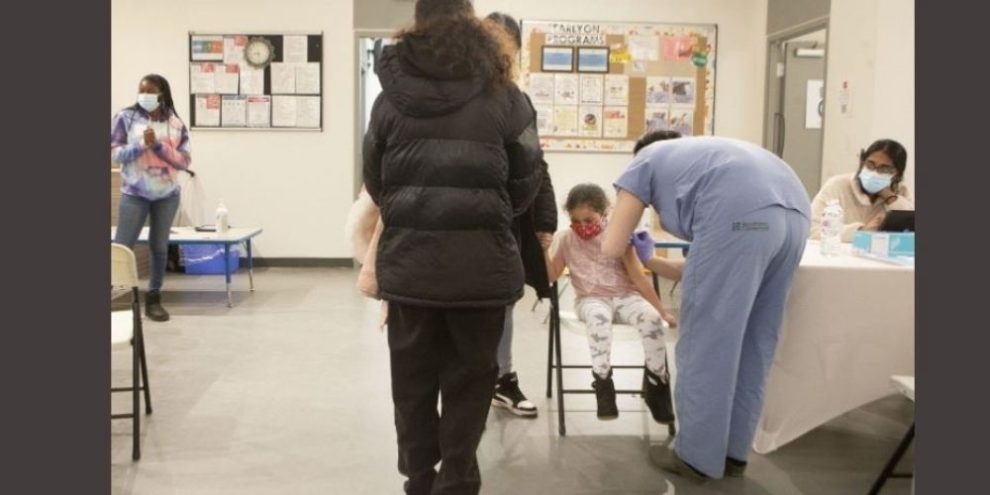
Banner image: Five-year-old Emma Palmer receives a COVID-19 vaccine at a "Kids and Families Vaccine Clinic" operated by Black Creek Community Health Centre and hosted by Jane and Finch Early ON child and Family Centre in the Jane and Finch Mall in Toronto on Thursday, January 13, 2022. THE CANADIAN PRESS/Chris Young
This report by The Canadian Press was first published April 29, 2022.






